Last updated: August 26, 2013
NIH researchers identify gene variant in Proteus syndrome

National Human Genome Research Institute
www.genome.gov
NIH researchers identify gene variant in Proteus syndrome
Molecular insight may confirm cause of the so called Elephant Man's severe disfigurement
Bethesda, Md., Wed., July 27, 2011 - A team of researchers has identified the genetic mutation that causes Proteus syndrome, a rare disorder in which tissue and bone grows massively out of proportion. The discovery, which has implications for potential drug therapies and even cancer, appears in the July 27, 2011, early online edition of The New England Journal of Medicine. The team was led by researchers at the National Human Genome Research Institute (NHGRI), part of the National Institutes of Health.
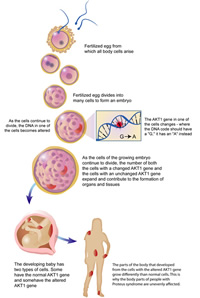
Proteus syndrome gained wide public attention in 1980, through the movie "The Elephant Man," about a man depicted as having the disease. Researchers found that a point mutation - a single-letter misspelling in the DNA of the genetic code - in the AKT1 gene activates the sporadic tissue growth characteristic of Proteus syndrome. Physicians named the condition for the Greek god who could transform his shape. There are fewer than 500 people with the disease in the developed world, where it can be tracked.
Unlike inherited disease-causing mutations, the gene variant that triggers Proteus occurs spontaneously in each affected individual during embryonic development. The severity of the disease depends on the timing during embryonic development that the genetic mistake occurs in a single cell and in which part of the developing organism. Only the cells that descend from the cell with the original AKT1 gene mutation display the hallmarks of the disease, leaving the individual with a mixture of normal and mutated cells.
The affected newborn appears normal, but symptoms arise in the child's first two years. The mutation in AKT1 alters the ability of affected cells to regulate their own growth, leading some parts of the patient's body to grow to abnormal and even enormous sizes, while other parts of the body remain normal. The irregular overgrowth worsens with age and increases the susceptibility to tumors.
"This study resolves a daunting challenge in clinical genetics and offers hope for patients with Proteus syndrome," said NHGRI Director Eric D. Green, M.D., Ph.D. "This rare disorder has been the focus of curiosity and medical observation for decades but until now has never been biologically explained. With the analysis reported here, patients and families who face this condition have hope for future therapies."
As follow up to the current study, NHGRI researchers plan to test DNA from the skeleton of Joseph Merrick to determine whether Proteus syndrome caused his dramatic disfigurement. Merrick gained celebrity - and for a time earned his livelihood in England and Europe - by being displayed in human novelty exhibitions as the Elephant Man. He died in 1890 at the age of 27 in London Hospital, now the Royal London Hospital, where he resided at the end of his life. The hospital preserved his skeleton in its pathology collection, providing modern researchers a chance to test his century old DNA. Merrick's life has been portrayed on stage, and in a 1980 Hollywood movie titled "The Elephant Man."
Diagnosing Merrick will be no simple study. Because of the way the mutation occurs during embryonic development, the NHGRI-led team found that the gene variant of Proteus syndrome occurs in only a subset of the body's cells rather than in every cell, a condition called genetic mosaicism. There are only a small number of known mosaic disorders in which an individual's cells have a different genetic composition from one another. Essentially, the person develops more than one genome. Since only a subset of the body's cells harbor the mutation, it is possible that during a medical biopsy, in which bits of tissue are cut out for analysis, the diagnosis may be missed because only normal cells are sampled.
"Diagnosis in our patients has been really difficult," said senior author Leslie Biesecker, M.D., chief of NHGRI's Genetic Diseases Research Branch. "This molecular discovery gives us a basis for objective molecular diagnosis for patients with perplexing forms of overgrowth."
Until now, clinical diagnosis has been based on observation of patient features. Besides overgrowth of limbs, the condition is characterized by a variety of skin lesions and thickening of the soles of the feet. Some patients have neurological complications, such as mental retardation, seizures and vision loss.
To find the single-letter misspelling among the 3 billion letters that make up the human genome, the researchers performed whole-exome sequencing on the DNA of seven patients with Proteus syndrome. Whole-exome sequencing determines the sequence of letters that make up the 1 to 2 percent of the genome that contains protein-coding genes. The research team then analyzed more than 20 additional affected individuals, finding the same gene variant in DNA in more than 90 percent of these individuals. The team suspects that the three individuals so far negative for the mutation may actually have the mutation at low levels or in different tissues than those sampled in the initial biopsy. By contrast, the variant is never found in unaffected people, including a random study population of more than 400 individuals and in thousands of DNA sequences maintained in public genome research databases.
The mutated gene, AKT1, is an oncogene, meaning that it can promote the kind of uncontrolled cell growth associated with cancer. The variant of AKT1 that causes Proteus syndrome is part of a cascade of mutations that also promotes metastasis, the process by which cancer cells spread to healthy parts of the body. AKT1 mutations have been detected in about two percent of cancer samples.
In cancer, an AKT1 mutation develops as part of a chain of mutation events that occurs in a limited number of normal cells of a particular organ of the body. In Proteus syndrome, because the mutation occurs in embryonic development, many more tissues of the body are impacted by the gene variant, though not all have overgrowth. According to Dr. Biesecker, a person could not survive if the variant that causes Proteus syndrome occurred so early as to be in all cells of the body.
Previous research demonstrated that the AKT1 mutation changes the cell growth-promoting activity of the AKT protein. NHGRI researchers found that cells from patients with Proteus syndrome had increased AKT activity at times when AKT would normally be inactive in unaffected individuals. The mutation acts like an accelerator of cell growth, but only in some tissues of the body.
To study the mosaicism affect of Proteus syndrome, the researchers tested cells derived from affected tissue and unaffected tissue of individuals with the disease. They analyzed the level of activation of AKT, confirming that affected tissue had increased AKT protein activity.
"We now have a better chance of making or finding a drug that can arrest this overgrowth and begin to use it early on in the disease progression," Dr. Biesecker said. "A factor in our favor is that it is much easier to find a drug that inhibits the activity of a protein, which is what we want to do with AKT in Proteus syndrome, than to activate a protein."
In the cancer field, there are a number of potential therapeutics being developed to inhibit the pathway involving this gene, some of them by inhibiting AKT1 itself. "For Proteus syndrome, AKT1 will likely need to be targeted for optimal benefit to affected patients," Dr. Biesecker said.
The researchers further demonstrated that tissue biopsies are required to genetically diagnose Proteus syndrome, since the variant that causes the AKT1 mutation is infrequently present in white blood cells typically sampled for genome analysis.
"During the past 15 years, Proteus syndrome patients have come to the NIH Clinical Center, where we have operated to help stop bones from overgrowing," Dr. Biesecker said. "Our tissue bank has grown during this period because we have been able to obtained samples of affected tissue during surgeries that we would otherwise not have had for this study. If we just asked pediatricians to mail to us blood samples of children with Proteus syndrome we would not have found the mutation. The NIH Clinical Center was essential in providing support and expert colleagues to allow us to do this research."
In addition to patients seen at NIH, the study included the efforts of collaborators from eight community hospitals and five university medical centers in four countries. Contributors to the study also included investigators from the NIH Intramural Sequencing Center, administered by NHGRI, and from the National Cancer Institute and the National Institute of Dental and Craniofacial Research. In addition to the hospitals and medical centers, the study authors also included representatives from two independent research groups and the Proteus Syndrome Foundations of the United States and the United Kingdom.
NHGRI is one of the 27 institutes and centers at the NIH, an agency of the Department of Health and Human Services. The NHGRI Division of Intramural Research develops and implements technology to understand, diagnose and treat genomic and genetic diseases. Additional information about NHGRI can be found at its website at www.genome.gov.
The National Institutes of Health - "The Nation's Medical Research Agency" - includes 27 institutes and centers, and is a component of the U.S. Department of Health and Human Services. It is the primary federal agency for conducting and supporting basic, clinical and translational medical research, and it investigates the causes, treatments, and cures for both common and rare diseases. For more, visit www.nih.gov.
Contact:
Raymond MacDougall
NHGRI
301-402-0911
macdougallr@mail.nih.gov