The NIH IRB specifies that "Beginning October 1, 2022, the NIH IRB expects new protocols to describe a plan for returning clinically actionable secondary genomic findings to research participants, unless there is a strong justification not to do so. The most commonly acceptable reasons for not returning results will be: 1) the absence of a clinical relationship with participants or 2) the data being generated by the study are insufficient for conducting secondary analyses."
All NIH intramural protocols that include human genomic DNA sequencing should consult the above policy. For more information see https://irbo.nih.gov/confluence/pages/viewpage.action?pageId=174162149 (NIH access only). Although the final decision lies with the IRB, Darnell et al. propose the following guidance:
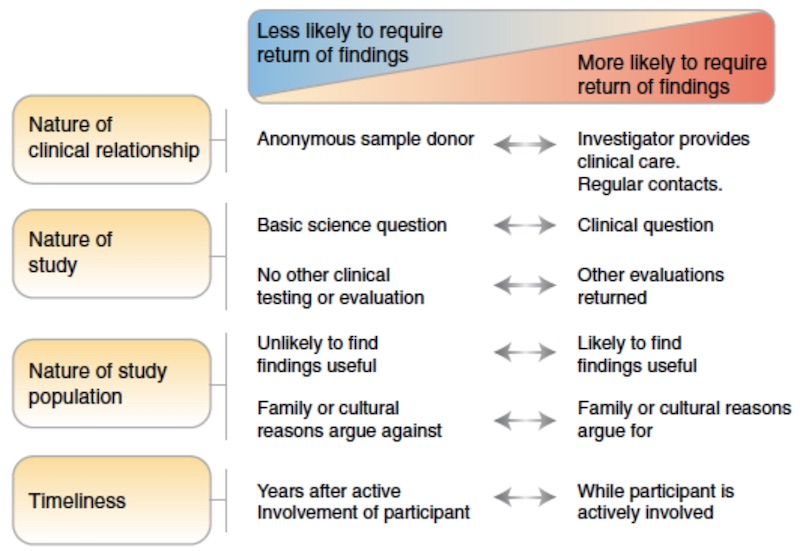
Currently, the American College of Medical Genetics recommends that variants in 81 genes be returned to people undergoing clinical sequencing, but there is no general or universal obligation for researchers to actively search for secondary findings. However, there are context-dependent obligations that researchers incur in the process of performing different kinds of research. The greater the degree that the participant-researcher interaction resembles a clinical care relationship, the greater the obligation of the researcher to return secondary findings that are of high clinical utility.
Returning secondary findings has the potential to improve health outcomes for your participants and their families. Moreover, multiple studies performed in diverse settings demonstrate that research participants, when asked, say that they would like to receive this kind of information.



