A protein’s unique flexibility may help it respond when viruses attack
An immune system protein is extremely adaptable. This flexibility has implications for public health and could inform the design of synthetic proteins.
If anything in nature is a well-oiled machine, it’s the cell. And if you pop open the hood of the cell, all those interlocking gears — constantly revolving to make the cell tick — are proteins. Let’s say all the gears have square teeth, but on one gear, the teeth suddenly become round or longer. Does the machine come to a screeching halt?
Changes in the DNA sequence (i.e., mutations) can alter a protein’s shape, and this can change or disrupt a protein’s function. But researchers at the National Human Genome Research Institute (NHGRI), part of NIH, found that a protein in the immune system is particularly adaptable to mutations.
“While mutations occur naturally across the genome over millions of years of evolution, most of your proteins don’t need to change,” said Meru Sadhu, Ph.D., an investigator at NHGRI and senior author of the study published in eLife. “However, proteins in the immune system have to adapt to a constantly changing environment, which makes the immune system a very interesting genetic space.”
The researchers zoomed in on a protein called PKR, which is found in nearly every cell in the human body. When cells become infected with a virus, PKR sends signals to alert the immune system to the virus’s presence.
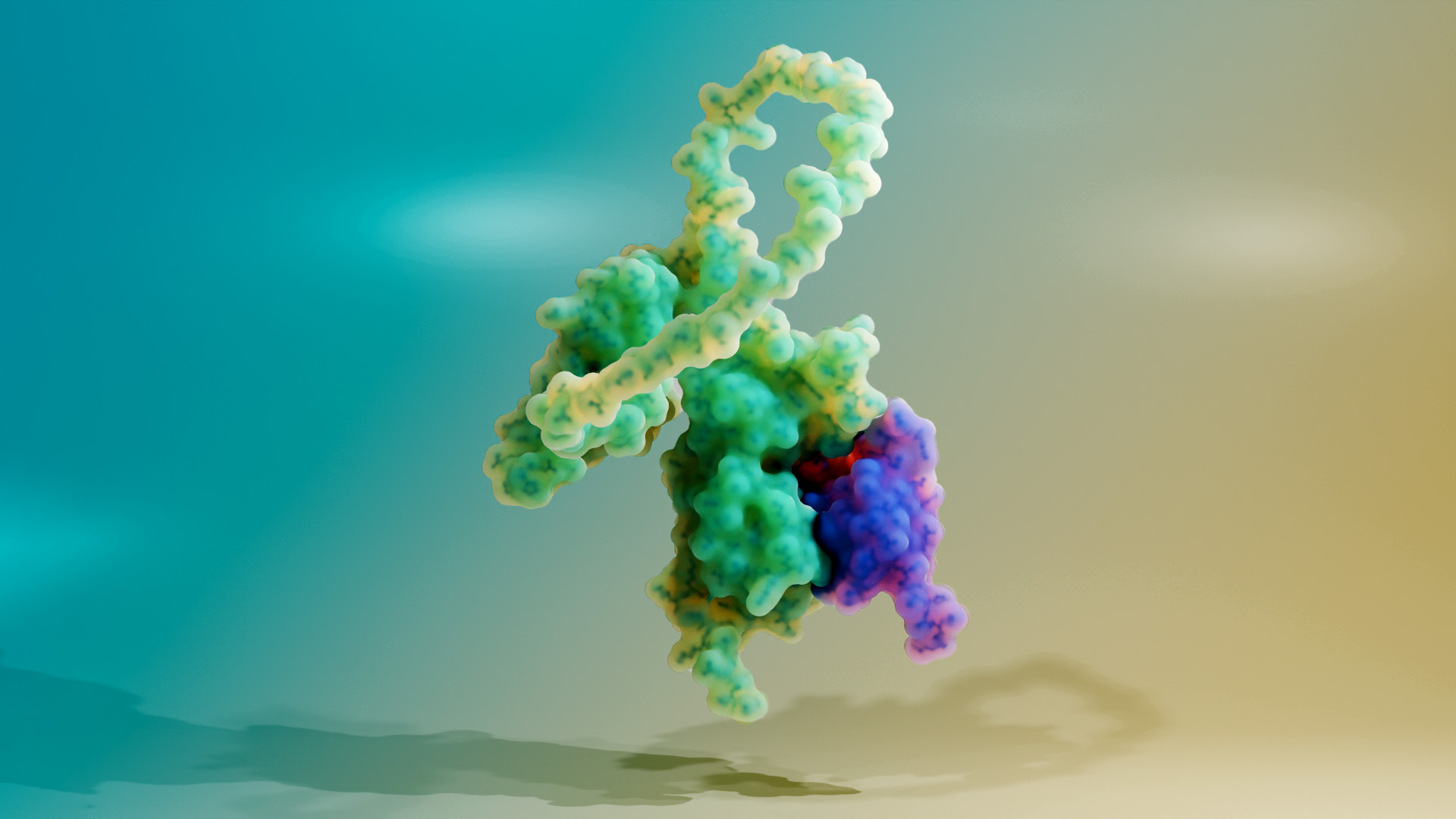
Caption: K3 (purple) binding to PKR (green).
However, viruses have different methods of shutting down PKR. Often, this involves a protein produced by the virus that binds to PKR, and the shape of each protein influences how well the proteins bind. Therefore, PKR, like other immune proteins, is under strong evolutionary pressures to change.
While mutations occur naturally across the genome over millions of years of evolution, most of your proteins don’t need to change. However, proteins in the immune system have to adapt to a constantly changing environment, which makes the immune system a very interesting genetic space.
To test which parts of the protein may be most adaptable, the researchers created over 400 variations of PKR by making specific changes to the gene’s DNA sequence. The researchers inserted the PKR variants into yeast, an organism commonly used in research. Studying PKR in yeast allowed the researchers to isolate this protein from the rest of the immune system and analyze the variants in a systematic way.
Very few of the variants disrupted PKR’s function. “I thought wow, it seems like this protein really tolerates a lot of mutations,” said Michael Chambers, Ph.D., a postdoctoral fellow at NHGRI who led the study. “Many other proteins would not be able to maintain function across this many changes.”
Several mutations did impede PKR function, and most of these were clustered in a few distinct locations on the protein. However, PKR tolerated changes in many regions that, like the teeth of a gear, are essential for its ability to perform its job.
“The big take home is that PKR is a very resilient protein,” Dr. Chambers said. “But that doesn’t mean every mutation is going to stop viral proteins from binding to it.”
The researchers also exposed the PKR variants to a viral protein called K3. This protein is produced by the vaccinia virus, a member of the pox family of viruses, which includes smallpox and the virus that causes mpox. K3 helps some pox viruses escape detection from the immune system by binding to PKR and stopping it from sending signals to the rest of the immune system.
The researchers found a significant number of PKR variants were both resistant to K3 and remained functional. Many of these mutations not only helped PKR fend off the regular K3 but were also effective against an extra strong version of the viral protein.
“We can see that PKR is really good at walking this tightrope, balancing between evading viruses and remaining functional, but the question that remains is how?” Dr. Chambers said.
More research needs to be done to understand how exactly different mutations change the protein’s shape. Additionally, the researchers only studied PKR’s response to one type of virus, but the immune proteins in our bodies — and those of other animals — have to fend off a variety of viruses throughout our lifetimes.
“Other work has studied PKR in different species, and we can see other species are taking fascinating approaches with this protein,” Dr. Chambers said. “Just look at bats.”
Bats have an second copy of PKR in their genomes. A virus may be able to shut down one copy of PKR but not the other. This may explain why bats can harbor so many kinds of viruses without becoming ill themselves, which is a major public health issue as these viruses sometimes jump to human populations.
“There also may be something to be learned from PKR’s structure that could inform the design of more flexible synthetic proteins in the future,” Dr. Chambers added.
Synthetic proteins are increasingly used for medical treatments and research purposes. Learning from PKR’s flexibility could lead to the development of more resilient synthetic proteins that could also be more easily modified for new applications.
About NHGRI and NIH
About the National Human Genome Research Institute (NHGRI): At NHGRI, we are focused on advances in genomics research. Building on our leadership role in the initial sequencing of the human genome, we collaborate with the world's scientific and medical communities to enhance genomic technologies that accelerate breakthroughs and improve lives. By empowering and expanding the field of genomics, we can benefit all of humankind. For more information about NHGRI and its programs, visit www.genome.gov.
About the National Institutes of Health (NIH): NIH, the nation's medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit www.nih.gov.
Press Contact
Last updated: December 9, 2024
